Deep Phenotyping of the Gut Immune System During Immune Checkpoint Inhibitor Therapy (DEFENCE)
Activation of the immune system by immune checkpoint inhibitors (ICI) results in long lasting anti-tumor activity in patients with various cancer types. Unfortunately, most patients fail to benefit from ICI therapy. [1] There is an unmet need to develop novel strategies to predict this response to ICI. The DEFENCE study is an ongoing, explorative clinical study aiming to define gut wall characteristics associated with response to immune checkpoint inhibitor (ICI) therapy. Here, we provide a brief overview of the study’s rationale and design.
The Gut Wall and Immune Checkpoint Inhibitor Therapy
Patients responding to ICI therapy have been shown to have higher peripheral and tumor-infiltrating CD8+ T cells than non-responders, both at baseline and during ICI therapy. The gut wall is a crucial immune organ, hosting over 70% of our immune cells. CTLA-4 and PD-1/PDL-1, the immune checkpoints that are the target of ICI, are present on immune cells in the gut wall and have an important function in the regulation of the intestinal immune system and maintenance of gut wall homeostasis. ICI therapy affects the gut, as evidenced by: a per treatment increase of fecal calprotectin – indicating intestinal immune activation, and shifts in intestinal immune cell subtypes, including CD8+ T cells, during ICI therapy. Furthermore, recent gut microbiome studies indicate that the tumor response to ICI can be modified by gut directed interventions. Surprisingly, very little attention has been paid to the gut wall in the context of ICI therapy. Thus, the effects of ICI therapy on the gut immune system are not fully understood. This study is the first aiming to study colonic immune infiltrates in relationship to the response to ICI therapy. [2]
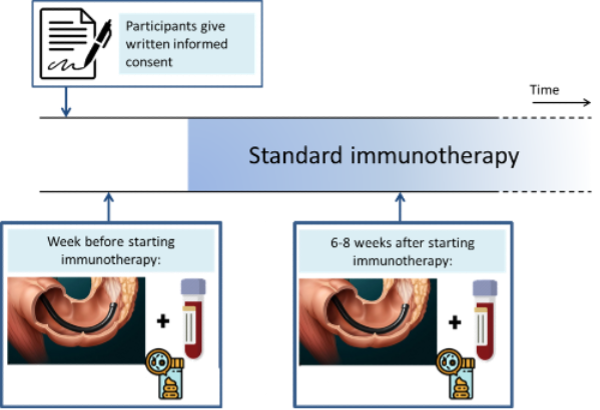
Study design
After informed consent is obtained, sigmoidoscopies are performed. Biopsies, for subsequent analyses of colonic immune infiltrates, are obtained from the sigmoid colon and rectum at baseline and 6-8 weeks after initiation of ICI therapy. (Figure 1)
All patients included are above 18 years of age, diagnosed with a metastatic solid malignancy evaluable according to RECIST v1.1., and have an indication for palliative treatment with anti-PD-1 therapy or dual anti-PD-1 and anti-CTLA-4 treatment. Exclusion criteria are: previous gastrointestinal resections, ileostomy or colostomy; abdominal radiotherapy within 6 months of starting ICI therapy; pregnancy; and the presence of abdominal symptoms such as pain, bloating or diarrhea that may indicate gastrointestinal inflammation.
Further details on the DEFENCE study can be found at clinicaltrials.gov (identifier NCT04600180).
References
[1] Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2:e192535.
[2] Hone Lopez S, Jalving M, Fehrmann RSN, Nagengast WB, de Vries EGE, & de Haan JJ. The gut wall’s potential as a partner for precision oncology in immune checkpoint treatment. Cancer Treat Rev. 2022;107:102406.
