Current Clinical Trials
Multiple clinical trials are ongoing in the Optical Molecular Imaging Group. The overview below shows which trials are currently running and their status.
VIDEO – Andrea Sterkenburg, Ymke van Ginkel and Henrik Huizinga
This study aims to investigate the distribution of ustekinumab. To achieve this, ustekinumab has been fluorescently labeled and will be administered intravenously to patients with CD or PsO.
Started in March 2025, currently enrolling patients.
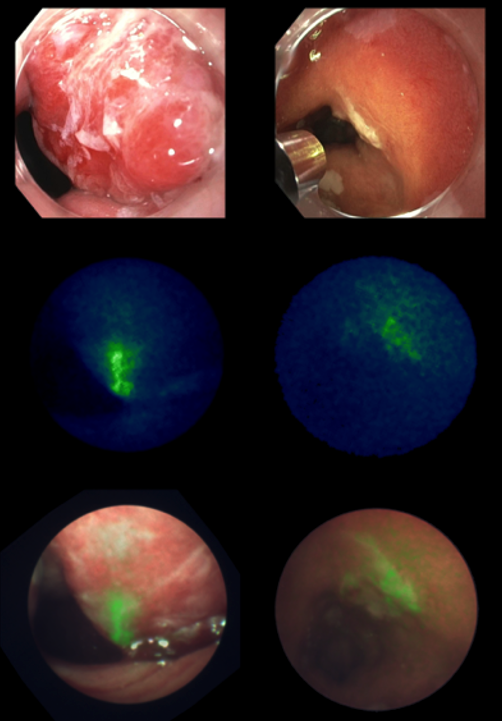
PREDICT – Pia Volkmer, Lisanne van Heijst, and Andrea Sterkenburg
In this study we employed fluorescently labeled versions of the anti-PD-L1 drug: durvalumab-680LT and the anti-PD-1 drug: nivolumab-800CW. This innovative approach enables direct measurement of PD-1/PD-L1 levels in vivo with dual-wavelength quantitative fluorescence molecular endoscopy (qFME).
Started in March 2025, currently enrolling patients.
NAVIGATE– Pia Volkmer, Rosalie Van Dijken , Henrik Huizinga, and Nawal Aledlbi
This study explores using fluorescently labeled risankizumab-800CW for quantitative fluorescence endoscopy to visualize local drug distribution and target cells, aiming to improve response prediction in IBD patients.
Started in November 2024, currently enrolling patients.
STRATIFY – Lisanne van Heijst and Ymke van Ginkel
This study explores the Near-infrared fluorescence molecular imaging of adalimumab-800CW to elucidate the drug distribution throughout inflamed tissue in inflammatory bowel disease and rheumatoid arthritis.
Started in August 2024, currently enrolling patients.
GUIDE – Pia Volkmer, Rosalie Van Dijken , Henrik Huizinga, and Nawal Aledlbi
This study explores using fluorescently labeled adalimumab-680LT for quantitative fluorescence endoscopy to visualize local drug distribution and target cells, aiming to improve response prediction in IBD patients.
Started in March 2024, currently enrolling patients.
In the DETOUR we will test the feasibility of immune optical coherence tomography (immune-OCT) in combination with fluorescence imaging (NIRF) with our targeted tracer bevacizumab-800CW.
Started in April 2024, currently enrolling patients
MARGIN – Bas Keizers and Joni Nijveldt
Improving radical resection rates in patients with breast cancer by intraoperative imaging using bevacizumab-IRDye800CW.
Started in January 2024, last patient included in January 2025.
SLURP – Lisanne van Heijst and Ymke van Ginkel
A phase II intervention study: detection of early esophageal neoplastic lesions by quantified fluorescence molecular endoscopy using oral administration of bevacizumab-800CW and cetuimab-800CW.
Started in August 2023, currently enrolling patients.
REFLECT – Thom Nijboer and Bas Keizers
Targeted fluorescence imaging using the fluorescent tracer Cetuximab-800CW to detect unknown primary tumours in the head and neck area.
Started in March 2023, currently enrolling patients.
LIGHTNING – Thom Nijboer and Bas Keizers
Targeted fluorescence imaging using the fluorescent tracer Cetuximab-800CW for tumour positive margin detection in patients with head and neck tumours.
Started in March 2023, last patient October 2024.
IMI-OPTIC – Anne van der Waaij, Sara Hone-Lopez, and Ymke van Ginkel
The IMI-OPTIC Study: Quantitative fluorescence molecular endoscopy using durvalumab-680LT to gain insight into the heterogeneity of programmed death-1 ligand (PD-L1) expression before and after neoadjuvant treatment in esophageal cancer.
Started in February 2023, currently enrolling patients.
LEAF – Pia Volkmer
Targeted fluorescence imaging using bevacizumab-800CW within neovascular Age-related Macular Degeneration (AMD) patients to evaluate the upregulation of VEGF.
Started in October 2022, last patient in March 2024.
SENSITIVE – Jouke van der Laan
An integrated approach of Raman spectroscopy and scattering measurements during endoscopy for real-time in vivo detection of field cancerization in the alimentary tract.
Started in July 2022, currently enrolling patients.
SELECT – Femke van der Zant and Eline Feitsma
Detection of tumor tissue in peritoneal metastases of colorectal origin using Bevacizumab-IRDye800CW during diagnostic laparoscopy
Started in May 2022, currently enrolling patients.
GLANS – Thom Nijboer
Visualization and resection margin assessment of penile carcinoma using the fluorescent tracer cetuximab-800CW and Surgvision camera system.
Started in March 2022, last patient in March 2023.
TARGET-Beva – Eline Feitsma
Margin and metastatic lymph node detection in patients with thyroid carcinoma, utilizing the fluorescent tracer Bevacizumab-IRDye800CW and the Quest Platform Camera system for in vivo imaging.
Started in December 2021, currently enrolling patients.
DEFENCE – Sara Hone-Lopez, Andrea Sterkenburg and Rosalie van Dijken
Studying the gut immune system and its changes during immunotherapy to understand the determinants of immunotherapy response.
Started in November 2021, currently enrolling patients.
LUMINA – Bianca Dijkstra
Evaluating the feasibility of applying bevacizumab-IRDye800CW for molecular fluorescence guided surgery in meningioma patients.
Started in November 2021, finished.
DEPARTURE – Iris Schmidt
The DEPARTURE study evaluates the feasibility of using intraoperative fluorescence imaging for the detection of bevacizumab-800CW accumulation in pituitary neuroendocrine tumor tissue.
Started in November 2020. Last patient in October 2022. Results published.
Utilizing Indocyanine Green (ICG) during total thyroidectomy for the purpose of parathyroid gland identification and preservation by means of fluorescence-guided surgery.
Started in November 2020. Last patient in September 2022.
TRACT-II – Anne van der Waaij and Wouter Hooghiemstra
Visualization of treatment response using cetuximab-800CW during fluorescence endoscopy in rectal cancer.
Started in November 2020. Last patient in January 2022.
VISION – Ruben Gabriëls and Anne van der Waaij
Phase I feasibility study investigating vedoluzimab distribution and detection vedoluzimab target cells in patient with inflammatory bowel disease.
Started in January 2020. Last patient in April 2022. Results published
ESCEND – Ruben Gabriëls and Wouter Hooghiemstra
Phase II near-infrared fluorescent endoscopy in combination with Bevacuzimab-IRDye800CW to visualize HD white light invisible dysplastic esophageal lesions.
Started in July 2019. Last patient in April 2022.
ORCA – Iris Schmidt and Anne van der Waaij
Fluorescence molecular endoscopy for response monitoring of esophageal cancer patients after neoadjuvant chemoradiotherapy using bevacizumab-800CW.
Started in November 2018. Last patient in September 2022. Results published.
COUGAR – Jouke van der Laan and Boudewijn de Vries
Perihilar cholangiocarcinoma detection using an intraoperative and endoscopic fluorescence guided approach using Bevacizumab-800CW.
Started in July 2018. Currently enrolling patients.