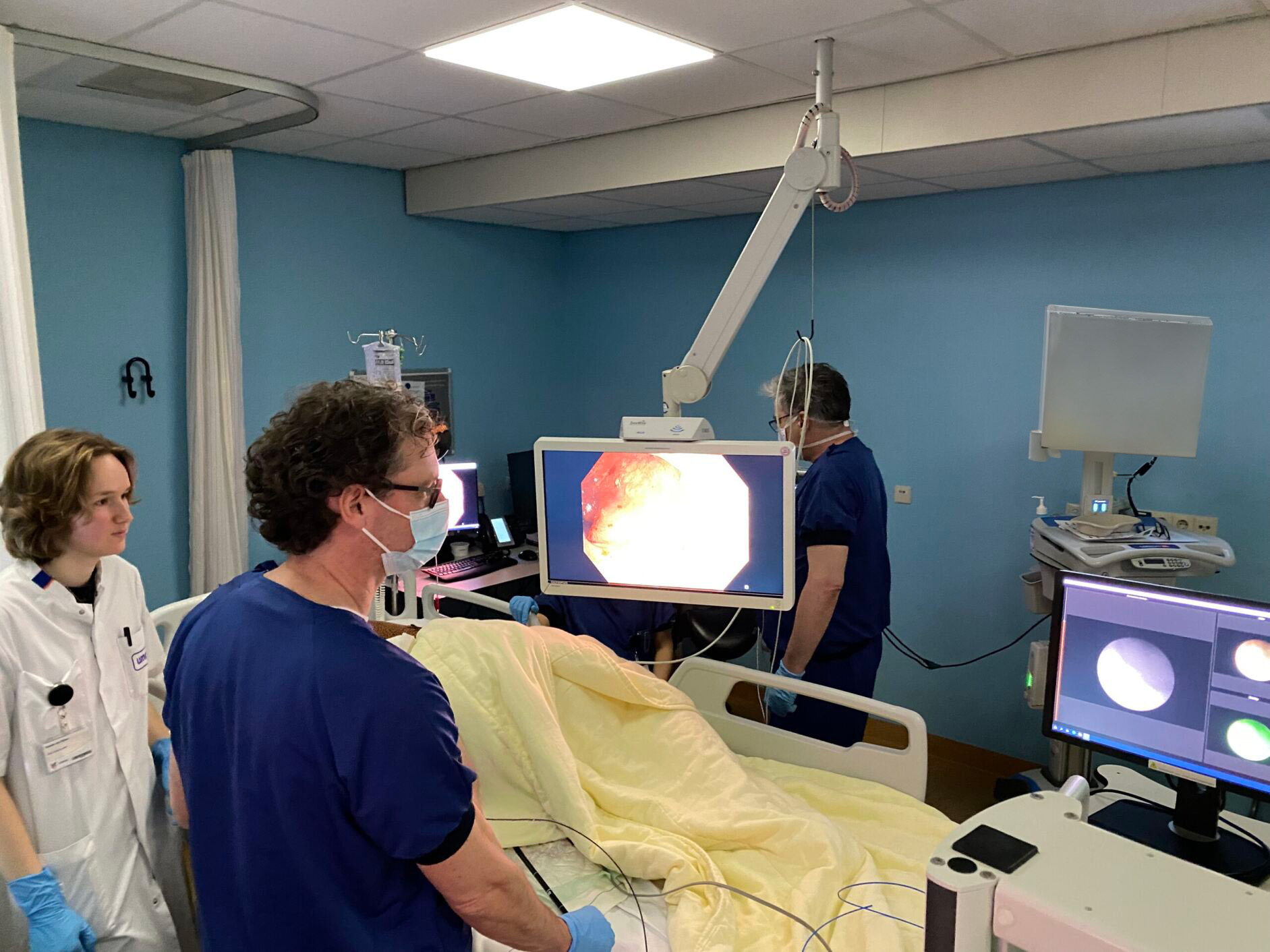
GUIDE: Fluorescently labeled Adalimumab-680LT for visualizing drug targeting in IBD
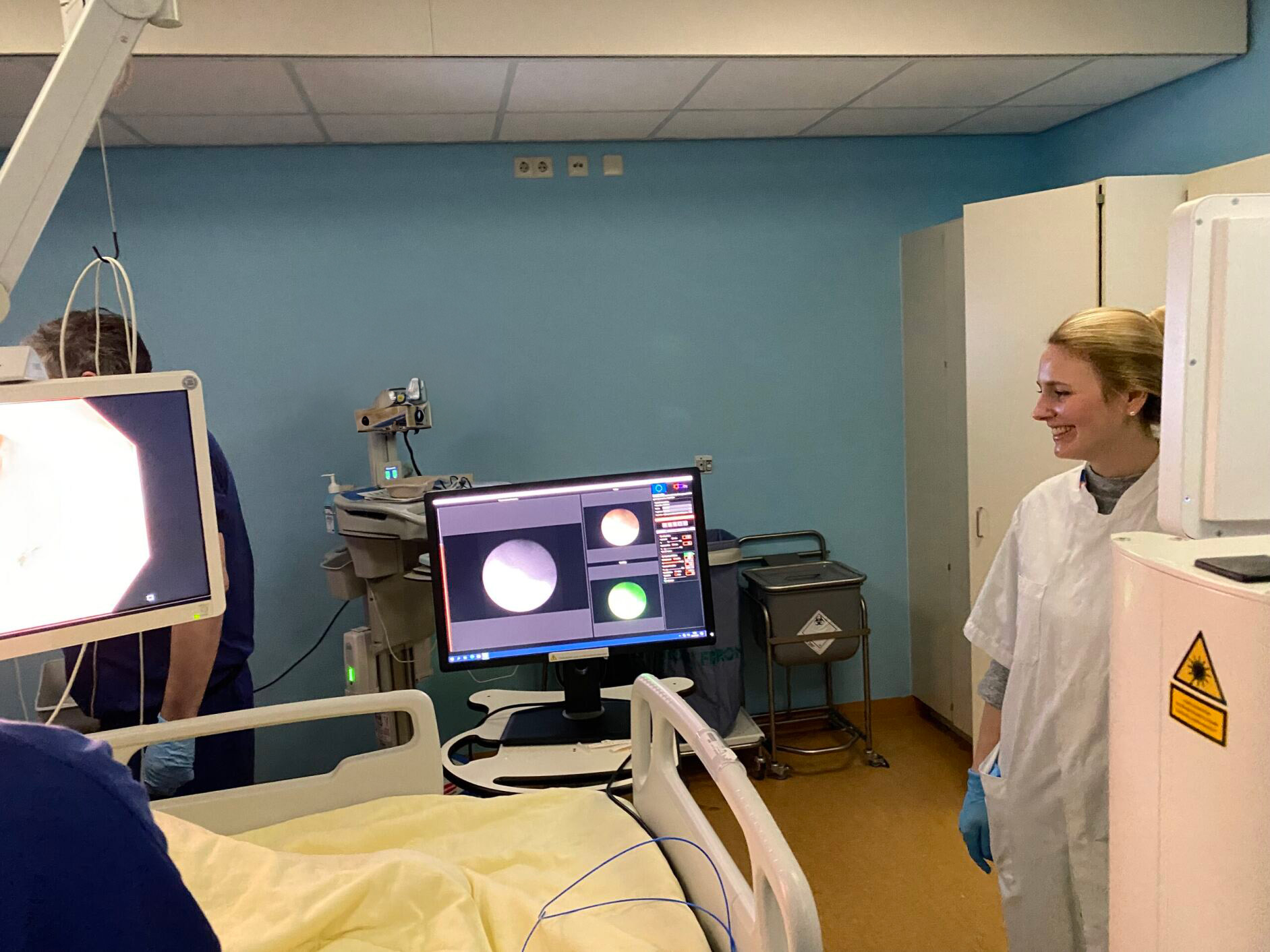
Biologic agents like adalimumab are often the last option for patients with inflammatory bowel disease (IBD) before surgical resection of the inflamed tissue. Unfortunately, many patients lose response to adalimumab over time, and we currently lack technologies that allow us to predict individual patients’ responses. In this study, we aim to investigate the feasibility of quantitative fluorescence endoscopy using adalimumab-680LT to gain insight into local drug concentrations and adalimumab target cells in IBD patients. We hope to use this method to predict treatment response to adalimumab in the future.
Background
Inflammatory bowel disease (IBD) poses significant challenges for patients and healthcare providers. Adalimumab is a monoclonal antibody in which a human constant region of immunoglobulin G (IgG1) is coupled to various regions of anti-TNF. Despite its widespread use for moderate to severe cases, it often falls short: up to 30% of patients don’t respond adequately during induction therapy, risking disease progression and experiencing potential side effects like allergic reactions, infections, and skin disorders. Additionally, up to 50% of patients face secondary loss of response within the first year of treatment, with 10% experiencing adverse reactions that necessitate discontinuation of anti-TNF therapy.
To address these challenges, our study aims to develop a predictive tool for assessing therapeutic responses in patients and understanding local drug concentrations before initiating anti-TNF therapy. To achieve this, we’re utilizing a fluorescently labeled version of adalimumab (adalimumab-680LT), allowing us to visualize and quantify drug distribution within tissue. This approach allows us to visualize and quantify drug distribution within tissue, offering promising prospects for improved treatment outcomes and personalized care strategies for IBD patients.
Study design
In this study, 18 IBD patients with active disease will receive varying doses of fluorescently labeled adalimumab-680LT before fluorescence molecular endoscopy (FME). Following this, ex vivo analyses will be conducted to correlate fluorescence signals with histological findings, providing valuable insights into tissue distribution and treatment response. Advanced techniques such as spatial transcriptomics will be employed to delve into cell composition inside the mucosa and the expression profile of adalimumab target cells. Additionally, by using tissue clearing followed by light-sheet/confocal microscopy, we can visualize the adalimumab-680LT distribution inside the mucosal biopsies in 3D and measure the actual concentration of the tracer.