An integrated approach of real-time in vivo Raman spectroscopy and scattering measurements for early detection of cancer onset based on sensing field cancerization in the alimentary tract during endoscopy (SENSITIVE)
Assessment of gastrointestinal field cancerization through Raman spectroscopy and scattering measurements
The presence of multiple tumors (multifocality) and tumor recurrence are associated with widespread changes in tissue areas extending far beyond the tumor site, which are described as tissue “fields”, including the microenvironment of the tumor areas. Microscale changes in cells and tissue throughout the whole organ occur as elements of field cancerization before neoplastic lesions become manifest in the gastrointestinal tract. Thus, detection of field cancerization in the tissue could prompt further endoscopic examination of the whole organ and allow real-time in vivo tissue diagnosis.
Through the implementation of Raman spectroscopy and scattering measurements in endoscopy, an approach is created that allows a label-free visualization of the smallest alterations in gastrointestinal (GI) tissue in a non-destructive manner. Raman spectroscopy assesses the biochemical composition of the tissue (the so-called ‘molecular fingerprint’ of tissue), whereas scattering measurements provide detailed information on tissue structure. Therefore, this integrated approach would be able to measure the intracellular processes and microarchitectural changes that are associated to field cancerization.
The SENSITIVE project
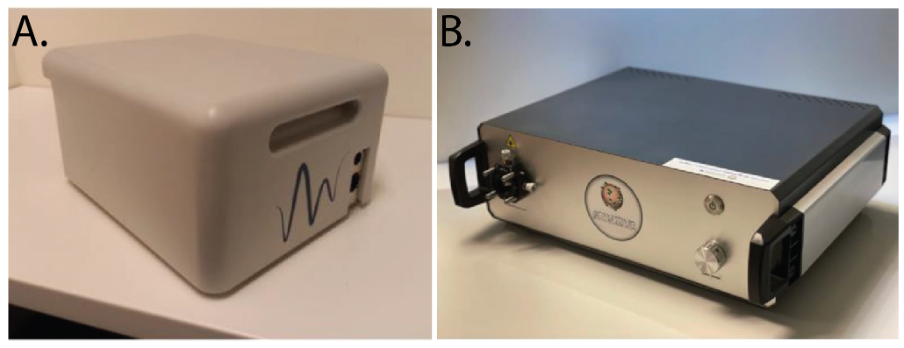
The SENSITIVE project is a 4-year term project that is funded by EU HORIZON 2020. SENSITIVE aims to investigate the feasibility of detecting field cancerization in the alimentary tract through an integrated approach of Raman spectroscopy and scattering measurements. The SENSITIVE project was initiated on November 01, 2018, and the first real-time in-human procedures in context of the clinical trial were performed on July 01, 2022. Prior to this final study, several pre-clinical and ex vivo studies were performed. During these studies, dedicated modalities for Raman spectroscopy (RiverD International, Rotterdam, The Netherlands – Figure 1A) and scattering measurements (Universidad Carlos III, Madrid, Spain – Figure 1B) for probe-based measurements during GI endoscopy have been developed, and extensive tests have been conducted to assess the quality and repeatability of the measurements and to select the optimal path for operating these modalities. Other research partners were involved during these phases as well, including BRFAA (Athens, Greece) and HelmholtZentrum (Munich, Germany).
More information regarding the SENSITIVE project can be found on: https://www.sensitiveproject.eu
Raman spectroscopy
Raman spectroscopy (RS) is based upon the inelastic scattering of photons that results from the Raman effect, in which the vibrational, rotational, or electronic energy of a molecule decreases or increases after excitation. As each molecule has a characteristic Raman signal, Raman spectra can be used to identify the molecular ‘fingerprint’ of a tissue.
Scattering measurements
By measuring the scattering properties, assessment of the structural changes in tissue and cells on a nanoscale can be performed. Therefore, structural alterations of field cancerization can be detected.
Aim
To assess the feasibility and safety of probe-based scattering measurements and (spontaneous) Raman spectroscopy to enable endoscopic real-time in vivo detection of field cancerization in the GI tract.
Study design
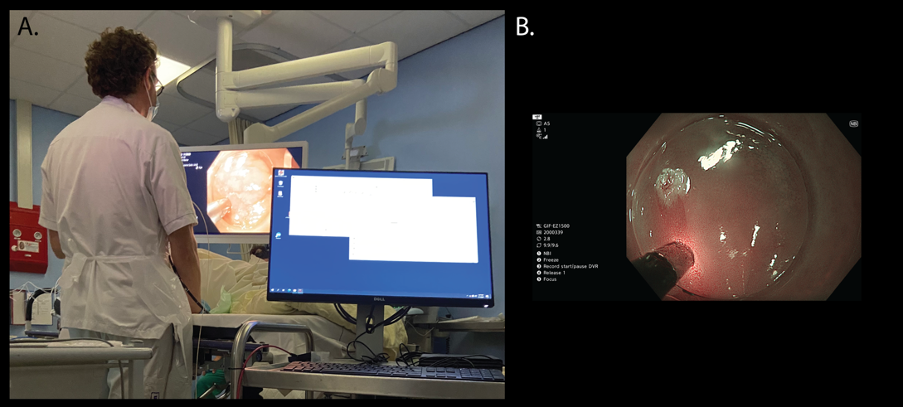
We aim to include a total 60 patients in two cohorts; 30 patients with Barrett’s esophagus (BE) and 30 patients that are at risk of developing CRC. From both cohorts, we aim to include 15 patients that have histopathologically established non-dysplastic tissue and 15 patients that do have a (pre-)malignant lesion in their esophagus or colon. Patients have a clinical indication for a gastroscopy or a colonoscopy due to either a diagnostic indication or therapeutic indication. Patients that have provided informed consent will undergo Raman and scattering measurements during endoscopy (Figure 2A-B). First, we will perform Raman measurements with the SRM1 device that was developed by RiverD International (Rotterdam, The Netherlands), and subsequently scattering measurements will be conducted using the modality that was developed by University Carlos III Madrid (Madrid, Spain). After measurements, targeted biopsies will be taken for ex vivo analysis.